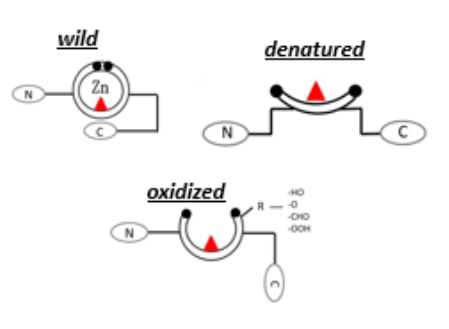
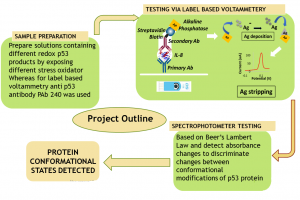
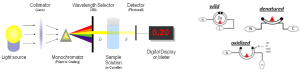
The development of sensing techniques able to perform an analytical evaluation of protein samples represents an area of great interest for biotechnology, pharmacology, and diagnostics. Traditional techniques available for protein quantification (e.g., ELISA) present limitations in terms of time and cost efficiency. On the contrary, optical-based techniques, such as spectrophotometry, might offer the interesting possibility to get information on the sample in a non-invasive way, allowing a continuous monitoring. This activity aims at developing a spectrophotometer-based method of identifying different conformational states of proteins, and in particular of p53, a redox sensitive protein involved in several pathophysiological processes. Samples containing different structural states of p53 protein can be investigated using spectrophotometer, in order to detect the differences in light absorption. The unfolding state of the different p53 modified redox products is further validated performing a label-based silver stripping voltammetry using the antibody PAb240, which is specific for the completely denatured p53 conformation. Preliminary studies allow to state the possibility to identify p53 different conformational states through this simple and non-invasive method, thus reducing the complexity of the procedures involved in conventional methods.
a
For more info, please contact Saad Abdullah, Mauro Serpelloni or Nicola Lopomo.a
a