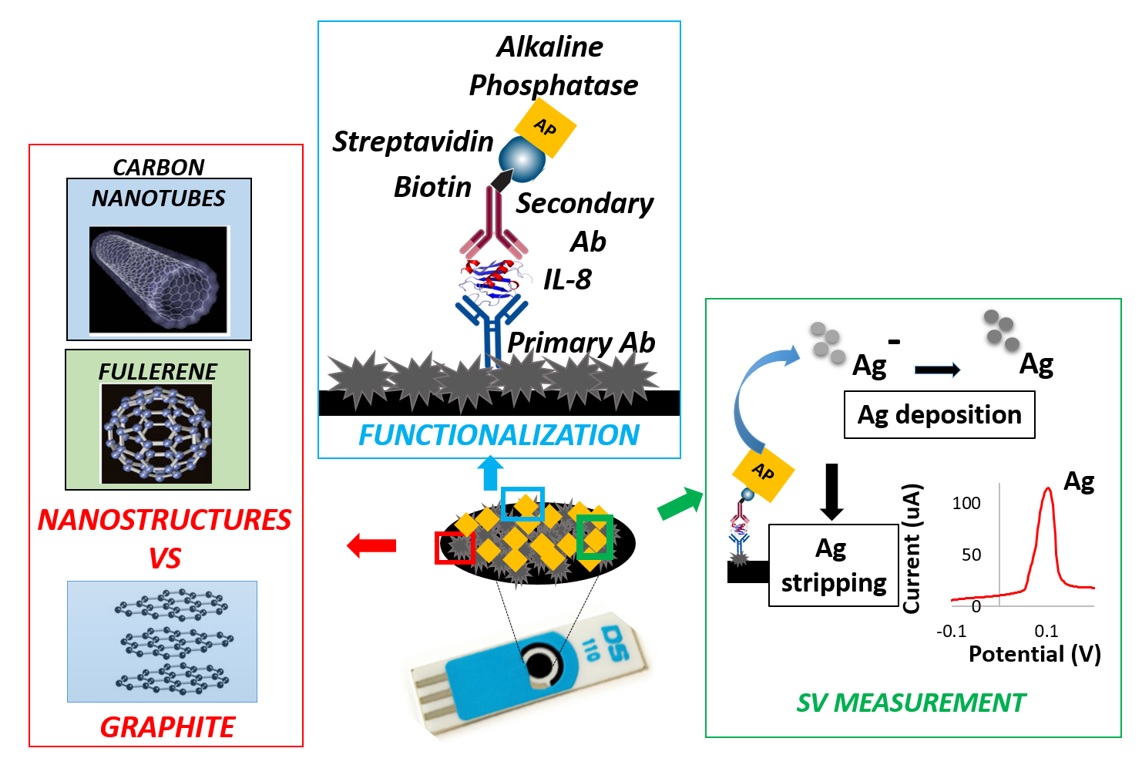
Detection and quantification of biomolecules inside biological fluids in the sub-nanomolar range is one of the most pervasive challenges in the field of pharmaceutical and medical research, in order to serve for early diagnosis of specific pathologies (e.g., cancer or neurodegenerative disease) or for the effective and non-invasive monitoring of various physio-pathological processes (e.g., inflammation). In order to obtain valuable information from biomolecules, an effective detection system must demonstrate high levels of sensitivity and selectivity, along with lower detection limits in biological fluids. Printed electronics represents a promising path to realize low cost, biocompatible, and highly sensitive sensors in order to correlate phases of biological phenomena with measurable changes in physical parameters. In particular, thanks to their low cost, ease of surface modification and miniaturization, Screen Printed Electrodes (SPEs) could represent a suitable solution to be integrated in complex and dedicated devices, thus obtaining fast, standardized and reliable electronic-based biomolecule quantifications, overcoming the limitation of currently available solutions. In light of this, the present research activity addresses, on one hand, the investigation of different nanostructured materials (e.g., Carbon Nanotubes, Platinum Nanostructures) and electrochemical techniques in order to improve the limit of detection for specific protein biomarkers using SPEs.
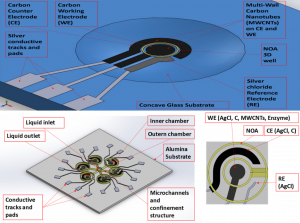
On the other hand, the research is directed in the development and production of a microsensor platform for the detection and quantification of proteins and other biomolecules, to be realized via aerosol jet printing. Sensors layout is designed, using the 3-electrodes configuration composed by working electrode (WE), counter electrode (CE), and reference electrode (RE) and commonly adopted for electrochemical measurements. The same geometry is reproduced as scaled in different dimensions. First results from geometrical and electrical tests suggest that the lines are microscopic with controllable thickness and proper electrical values coherent with each ink. Furthermore, imaging findings confirm good adhesion of antibodies on sensors WE, whereas ASV measurements highlight the possibility to effectively use the sensors for quantifying protein in a sample. This research is on course, through the implementation of successive steps, which involve the use of different substrates, such as glass and alumina, and a photopolymer to realize containment structures and microfluidics channels. In addition, the possibility to consider other applications for the platform, such as glucose sensing, is under investigation.
a
A
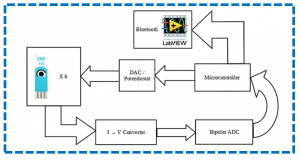
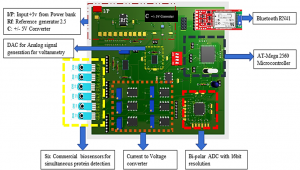
The final aim of the activity is developing a low-cost portable point-of-care testing system for early biomolecule detection. Such system is conceived as a multichannel potentiostat, which operates by regulating the potential difference between RE and WE to excite the biosensor for the detection. The working electrode receives potential from the potentiostat, leading to a decrease or increase in the number of electrons. As a result, triggering of biomolecule occurs and potentiostat records this change to measure the presence and type of the biomolecule within a solution, hence providing an innovative multichannel device with simultaneous multiple readout units.
a
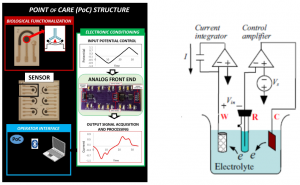
Overview of the Point of Care platform (left) and electrical model for a detection system (potentiostat, right).a
a
The activity is carried out in collaboration with the Department of Molecular and Translational Medicine at the University of Brescia and with the Escola d’Enginyeria de Telecomunicació i Aeroespacial de Castelldefels (EETAC), Universitat Politècnica de Catalunya, Castelldefels (Spain).
a
For more info, please contact Edoardo Cantù (for aerosol jet-printed sensors), Saad Abdullah (potentiostat), Sarah Tonello (SPEs), Mauro Serpelloni or Nicola Lopomo.